Clinical Trials
The US FDA’s advisory panel on general and plastic surgery devices voted in favor of agency approval for a novel medical device for treating early stage, low-risk breast cancer as a potential alternative to lumpectomy. The agency is expected to make its decision on the device early next year.

In this week’s Digital Health Roundup, Medtech Insight’s Marion Webb talks about her interview with GE HealthCare’s chief AI officer Parminder Bhatia about his vision for AI in health care and other highlights from HLTH. Natasha Barrow discusses her interview with Owkin on the EU AI Act and highlights Click Therapeutics’ latest clinical results.

Patients treated with Abbott’s Esprit BTK system had better results and fewer repeat procedures after two years than a control group, the company announced this week. Abbott vascular leader Jennifer Jones-McMeans spoke with Medtech Insight about what the results could mean for patient care.

The US FDA’s Patient Engagement Advisory Committee met Wednesday to discuss ways the agency can empower patients thinking about whether to participate in clinical trials of regulated medical products. Central to the committee’s discussion was not only the type of information patients need to evaluate when considering joining a study, but how that information should be presented to them.

The US FDA has published a final guidance document providing stakeholders with a framework for various safety evaluations they should consider when developing medical products for newborns, including devices. The guidance focuses on long-term evaluations of neurodevelopmental safety.

Oxford Medical Products (OMP) announced positive results from its first randomized controlled trial of weight loss device Sirona, an inert dual-polymer hydrogel pill that expands in the stomach to mechanically suppress appetite. CEO Camilla Easter views the device as complementary and potentially synergistic with GLP-1 receptor agonists “at a fraction of the cost.”
The medical device industry supports the FDA's draft guidance document on Diversity Action Plans but seeks flexibility and clarity, especially for international and IVD trials, and recommends using real-world data for postmarket studies.

Start-ups pitched a diverse deck of innovative technologies to three judges and an audience of potential investors, strategics and physicians at the Octane Medical Innovation Forum in Irvine, CA. Highlights include neuromodulation company Sinaptica Therapeutics, which won the competition for both “People’s Choice” and “Judge’s Choice.”

This week, J&J announced that it was buying heart failure device firm V-Wave; Procept got the FDA’s OK on a clinical trial of its Aquablation treatment for prostate cancer; and CMS began to consider Medicare reimbursement of Abbott’s TriClip tricuspid repair device.

Marabio Systems says the new funding will help accelerate efforts to bring a blood test to market in 2025 that will accurately determine if a mother is a carrier of antibodies that cause MARA, a subtype of autism that believe to cause more severe behavior.

The US FDA’s Office of Women’s Health provides a research roadmap to address health concerns specific to women. The FDA recently updated the roadmap, outlining areas in which further research is needed.
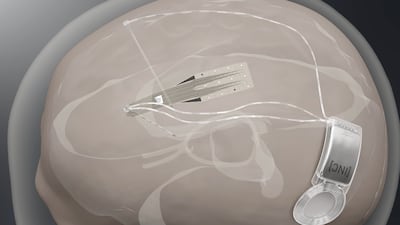
Spain-based Inbrain Neuroelectronics plans first-in-human study to show safety of its graphene-based technology in direct contact with human brain while also developing a second interface for treating Parkinson’s disease.
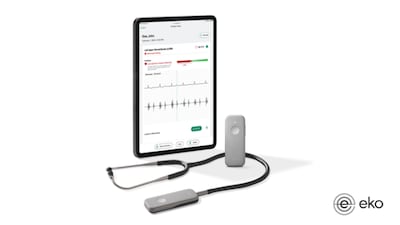
The results from a trio of studies show that AI-powered digital stethoscopes are effective at identifying patients at elevated risk of experiencing heart attacks and other major cardiac adverse events, according to Eko Health, whose technology was used to screen the participants in the studies.

The ISRCTN clinical trials registry has launched an improved dashboard to provide metrics that reveal how many studies are complying with key transparency requirements. Badges are in place for individual studies meeting the transparency criteria.
After announcing positive results showing that its Stentrode BCI is safe in six patients, brain-computer interface company Synchron is planning a pivotal trial to eventually file for FDA approval.

During an online seminar hosted by the Alliance for a Stronger FDA, Commissioner Robert Califf discussed key issues facing the agency, including supply chains, device shortages, and the risk of another pandemic.

Altoida CEO Marc Jones spoke with Medtech Insight about the company’s investigational digital screening tool for Alzheimer’s and the dire need for better, more accessible precision neurology diagnostics as the global population ages, neurologist shortages worsen, and groundbreaking Alzheimer’s drugs change the treatment paradigm.
TRiCares announced first implantation of the Topaz transfemoral tricuspid heart valve replacement system as part of the company’s EU pivotal study. If all goes to plan, the device will compete with Edwards’ Evoque system. The announcement follows the company’s $50m series D funding raise in July.

Patient centricity, diversity, innovation and transparency are key topics in the World Health Organization’s new guidance that recommends best practices for clinical trials and complements existing international guidance in this area.
According to Zacks Equity Research, the new trial seeks to support AlphaVac’s adoption in the European market, where pulmonary embolism prevalence and severity is an estimated 435,000 events annually.