Manufacturing

The new company, Rhine Pharma, hopes to avoid the isotope production issues that have dogged other radiopharmaceutical developers.

Breaking with convention, Thomas Schinecker has said allowing the $16.5bn acquisition to go ahead would be “the wrong decision”. Novo Nordisk says otherwise.

In a video interview, Nandini Piramal, chairperson of Piramal Pharma, speaks with Scrip about a focus on on-patent products, an integrated ADC offering, a measured approach to the proposed BIOSECURE Act and the company’s strategy to double revenue to about $2bn
Emerging Company Profile: The Maryland-based biotech is working on a functional cure for type 1 diabetes that is similar to Vertex’s VX-880 – but with some key differences.

Indian CMOs, CDMOs and CRDMOs are expanding capacity and making leadership moves ahead of the BIOSECURE Act, anticipating demand from big pharma and budding biotechs. Scrip brings details on leading Indian firms which could be potential go-to suppliers for US companies affected by the proposed Act

The drug maker will spend $4.5bn on a “Lilly Medicine Foundry” that it said would provide extra manufacturing capacity as well as research ways to improve manufacturing processes.

In a video interview with Scrip, Syed Husain, CEO of the US-based cell therapy CDMO BioCentriq, talks about the company’s role in parent GC’s growth strategy and business priorities, the cell and gene therapy manufacturing market and his views on the US BIOSECURE Act.

The biotechnology and pharmaceutical sectors outsource much of their drug discovery and clinical development efforts to contract organizations. Investors’ reduced appetite for biotech is now impacting those contractors.
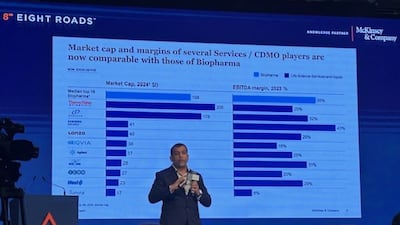
A redistribution of pharma outsourcing activities, both ex-China and also a wider shift in CDMO work from North America down the line may be in store. A senior McKinsey executive discusses with Scrip these trends in the CDMO sector amid geopolitical tensions, the spurt in customer queries at Indian firms and also facets of the deals scenario.

Does Zydus’ 50% stake acquisition in Sterling Biotech reflect a broader move of formulations companies to secure API supplies or acceleration of API segment restructuring begun by a couple of private equity (PE) funds?

The company is offering Zepbound in single-dose 2.5mg and 5mg vials, both to expand supply and access, especially for patients without coverage for the obesity drug.

Serum Institute is working on an mpox vaccine while other Indian firms are weighing options after the WHO sounded an alert and a new case was reported in neighboring Pakistan. Scrip looks at factors that could favor development and/or manufacture of an mpox vaccine at Indian majors.

Linvoseltamab has missed out on an August US approval, but Regeneron remains confident it will emerge as the best-in-class BCMAxCD3 bispecific therapy.
The company will ramp up manufacturing of its Jynneos vaccine to meet demand in worst-affected African countries and stockpiling richer nations.

Sales of Wegovy and Ozempic were strong again in the second quarter and while they were shy of analyst expectations, the Copenhagen-based giant is confident of continuing demand despite competition from Eli Lilly’s Zepbound.

Amid deals activity in India, Torrent Pharma executives spelt out their acquisition expectations and licensing strategy while an in-licensed Takeda brand did well in Q1 of FY25. The US growth trajectory is seen determined by five to ten product approvals expected this fiscal

The Swiss contractor said it will invest about $981m to increase manufacturing capacity for GLP-1 peptides in the US and EU, to fulfill API contracts worth more than $3bn.

The Madrid-headquartered group could see its finances transformed with a sale of its third party manufacturing services which have blossomed through its partnership with Moderna.

SK Bioscience’s acquisition of top 10 CDMO marks first major acquisition as part of its growth acceleration strategy and the biggest such investment so far by a Korean vaccines firm. Execs say deal is aligned with SK Group’s wider moves to 'rebalance' its businesses.

SanBio’s lead cell therapy asset has been on a bumpy journey to its global-first approval and while a nod has now come in Japan in a high-need indication, a commercial launch is conditional on additional data to establish product equivalence and manufacturing consistency.