
Retail

California authorities say predominance of retailers licensed to sell hemp are compliant with ban published effective on 3 October through emergency regulation. But hemp industry’s contesting the regulation in state court, arguing state authorities inappropriately used emergency action to change state law on hemp products legislature passed in 2021.

Accelerating new store openings and adding 6 million more loyalty members are among Ulta’s newly unveiled strategic priorities. At the firm’s 16 October Investor Day, the retailer reported it is lowering its long-term outlook to account for “normalization” of the US beauty market post-COVID and increasing prestige competition.

Jessica Spence joins as North American president while CFO Dan Sullivan becomes COO and Francesca Weissman, finance and business strategy SVP, adds CFO to her title in latest executive office changes by the marketer of brands including Schick men's and women’s shaving, Playtex, Stayfree, Carefree and o.b. feminine care and Banana Boat and Hawaiian Tropic sun care.

Nonbinding forecast, fourth for the program, includes risks associated with codeine-containing cough medicine as a topic FDA will include in ongoing evaluation of GRASE for pediatric cough cold drug products marketed under monograph for cold, cough, allergy, bronchodilator and anti-asthmatic OTC drugs.
Democrats Wyden and Merkley author Cannabinoid Safety and Regulation Act to limit sales to consumers 21 and older and authorize FDA to order recalls and impose bans on cannabis products with dangerous chemicals or additives. It also would establish regulatory structure for using cannabinoids found naturally in hemp and allowing “semi-synthetic” ingredients while prohibiting artificial or fully synthetic cannabinoids.

In addition to commentary and information about the OTC drug, dietary supplement and cosmetic industries, speakers offered candid remarks on subjects well known across the consumer health industry or well recognized by consumers.

Bayer’s Midol and nonprofit Period. provide PeriodTalk campaign for awareness of the gaps in period education; “The M Factor” film coming day before World Menopause Day in October; Exeltis USA’s Blues Away provides postpartum mood support; and Doctor's Best Women's Collection includes menopause, sleep, digestive, heart and hair/skin/nails support products.
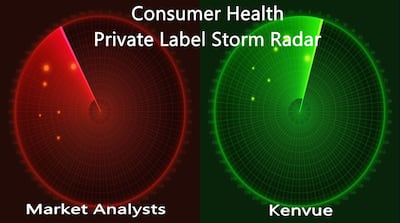
“We have a relatively low exposure to private label to start with. But we don't see penetration of private label going up in 2024,” says CEO Thibaut Mongon. Recent research from analysts, though, shows increase in consumers turning to lower-priced health and personal care alternatives.

The Bolingbrook, Ill.-based beauty retailer cuts its fiscal full year 2025 guidance, citing shoppers’ migration to value-based products and competitive intensity, among other factors. The firm reported net sales for the fiscal second quarter inched up 0.9% to $2.6bn.

Laifen, Opalescence Moon, Lewie and quip are among US companies with recent launches to boost their oral care market shares. Recent launch by Arm & Hammer global oral care brand also targets driving growth in the category.

Wild.AI women's health and wellness app available on Zepp’s Amazfit Active; WAT Medical’s app helps users lose weight; and firm Cronometer adds feature to answer users’ most frequent question, "How do I track water?"

Angelina Jolie For TOM FORD; Haus Labs by Lady Gaga; Gillette Targets Gamers; Cosmetic News In Brief
Estee Lauder Companies’ TOM FORD signs Academy Award-winning actress Angelina Jolie as the face of its Runway Lip Color Campaign launching 3 September. Dolly Parton introduces her first beauty range, Dolly Beauty, through Scent Beauty. More cosmetics and personal-care news in brief.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.

Mentholatum says two line extensions made with 2% salicylic acid and its "Oxy for Every Kind of Ne" campaign reinforce the brand’s “commitment to tackling every type of acne—from face-ne and chin-ne to body-ne.”

“We continue to believe that taking care of your health or someone in your family is the last place to look to make a change or save a few pennies,” says CEO Ron Lombardi. Firm more frequently using air freight services so Clear Eyes products are on store shelves when consumers expect.

L’Oréal's European business recorded sales growth of 9.7% to €3.55bn in the second quarter as consumer confidence grows, while in the US sales slowed for the dermatological beauty business and consumers in China are value-shopping.

P&G's FY2024 Q4 net sales were flat at $20.5bn, lower than consensus estimates, but the firm says its underlying business divisions are healthy and forecasts FY2025 sales up 2% to 4%, up 3% to 5% organically.

FDA reminds companies which registered with agency solely to manufacture OTC sanitizers during COVID-19 public health emergency they will be subject to FY2025 OTC monograph user fees if they don’t delist and deregister as monograph drug manufacturers by 12 a.m. on 31 December.

House and Senate reports again reference sunscreen but add admonishment that FDA “harmonize its approach with international testing standards.” Item exclusive to House report encourages guidance for drug firms on successfully submitting an application for Rx-to-OTC switch of an oral contraceptive.

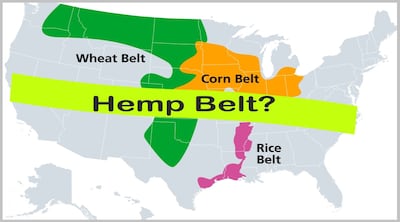
Committee’s report published with FY2025 appropriation states a different approach to establishing FDA regulation of non-drug products containing hemp as a derivative of cannabis de-scheduled as controlled substance in the US since 2018.