ADVERTISEMENT
OTC Devices
FDA’s OTC office director details a 2023 guidance as opening doors for NRT innovation at recent public meeting, but researchers and an industry executive note the most recent approval in the US for an innovative NRT was more than 20 years ago and say FDA isn’t allowing sufficient flexibility for approvals of new products or indications other than cessation related to quitting smoking.

FDA tobacco programs chief emphasizes moving smokers to lower risk alternatives and NIDA executive encourages proposals for e-cigarettes as nicotine replacement treatments. FDA also seizes $76m in unauthorized e-cigarettes.

Qnovia notes NRT inhalation product recently received investigational new drug clearance from FDA as agency and NIH say innovation needed smoking cessation to help improve rate of success for quitting the habit that kills around 500,000 US consumers annually.

This week, J&J announced that it was buying heart failure device firm V-Wave; Procept got the FDA’s OK on a clinical trial of its Aquablation treatment for prostate cancer; and CMS began to consider Medicare reimbursement of Abbott’s TriClip tricuspid repair device.
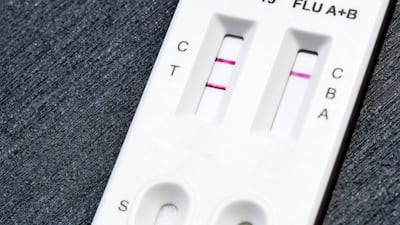
FDA grants de novo authorization to Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first OTC flu test to be cleared outside the emergency use pathway.
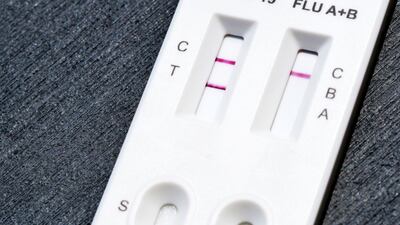
The US Food and Drug Administration has granted a de novo authorization to the Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first over-the-counter flu test to be cleared outside the emergency use pathway.

To coincide with Stoptober – an annual campaign that encourages smokers in the UK to quit smoking for the month of October – Kenvue has launched a digital “branded entertainment” series for smoking cessation brand Nicorette that follows smokers on their quitting journeys.

Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”

Use of Kenvue’s Nicorette QuickMist nicotine replacement therapy device resulted in “statistically significant reductions in urges to vape as soon as 30 seconds and up to two hours after dosing compared with placebo,” according to a recently published study funded by the firm.
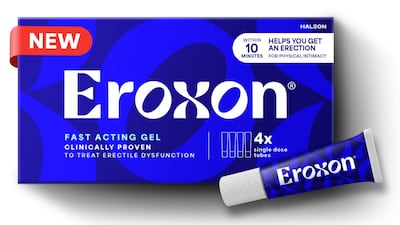
October will see the bricks-and-mortar launch of the first FDA-approved OTC treatment for erectile dysfunction in the US by Haleon. HBW Insight catches up with the company's North America president Lisa Paley to find out what Haleon has planned for Eroxon's arrival in stores.