ADVERTISEMENT
Commercial
Octave Bioscience’s CEO Doug Biehn sat down with Medtech Insight at HTLH to talk about the company’s plans for expanding their multivariate biomarker blood test for MS analysis and development of a diagnostic for Parkinson’s disease.

The Spanish company's Lilly-partnered atopic dermatitis drug is enjoying a decent launch in Germany and in other European countries, "we are getting the price that we wanted," claims CEO Carlos Gallardo.

An interactive look at recent executive-level company changes and promotions in the biopharma, medical device and diagnostics industries.

Experts working in the advanced therapy sector tell In Vivo how novel solutions can empower cell and gene therapy manufacturers to reduce costs, improve scalability and optimize their processes – improving the clinical profile and commercial viability of products.

Medixci’s Nick Williams told attendees at BIO-Europe that it was a shame that promising biotechs head to the NASDAQ as soon as possible. He and called for a change in culture from investors and for them to back the continent’s start-ups with proper funding.

The Denmark-based major intends to build on its heritage in diabetes and is keen to hear from innovators in a space where prevalence is on the rise but is not being served by improved new products.

While Japanese firms still dominate the money spinners in Asia, companies from China and, lesser so, India are starting to stand out from the crowd.
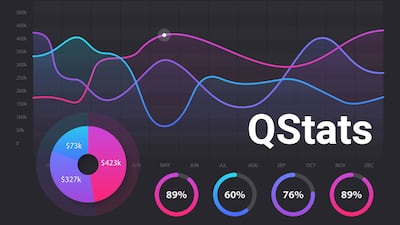
During Q3, biopharma merger and acquisition deal value reached $11.7bn and drew in $33.1bn in potential deal value from alliances. Device company M&A values reached $8.2bn, while in vitro diagnostics and research tools players’ M&A activity totaled $86.3m.

A wealth of new therapies are set to successfully graduate from the pipeline in 2025. Within this cohort are a mixture of therapeutic areas, drug classes, first-time approvals, label expansions, and treatments that will meaningfully change how diseases are treated.

All eyes are on Eylea, after Amgen said last week that it would launch the first US biosimilar in the face of ongoing litigation. The California-based player has spoken of its prospects in the wet-AMD space, including a desire to chart its own path amid supply shortages for Avastin.