
Diabetic Care
The company has taken the ambitious approach of establishing a manufacturing base capable of producing millions of devices before any attempt at a regulatory submission.

Beta Bionics’ automated insulin delivery system is now pairable with Abbott’s FreeStyle Libre 3 Plus CGM.

In her inaugural address to the medtech industry during the Medtech Conference, acting US CDRH director Michelle Tarver described her mission-driven approach.

This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.

Highlights from Medtech Insight's on-the-ground coverage of LSX in Boston.

This week, a Delaware court awarded Auris Health shareholders $1bn in a lawsuit against Johnson & Johnson; Abbott recalled some FreeStyle Libre 3 sensors; and McKesson purchased a controlling interest in a Florida cancer care chain.

Dexcom announced the launch of Stelo, the first non-prescription continuous glucose monitoring system to hit the US market, but is likely going to face competition soon from Abbott.

Despite a March recall and a following update, Tandem’s t:slim X2 application is still causing the battery-depleting defect.

Medtronic and Abbott announced partnership to integrate Abbott’s CGM sensor with Medtronic’s insulin pump, which marks a major departure from Medtronic’s former “closed-system” approach. Medtronic also announced FDA clearance of its Simplera CGM, paving the way for its use in smartpens.

Medway NHS Foundation Trust joined forces with Digostics to offer home oral glucose tolerance testing for pregnant women at risk of gestational diabetes.
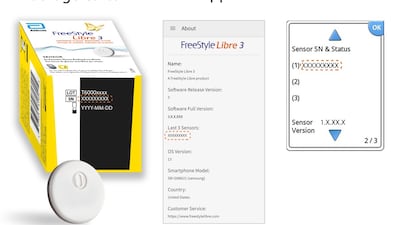
Abbott has issued an urgent voluntary medical device correction for a small number of FreeStyle Libre 3 sensors distributed in the US during May. The sensors could give inaccurate readings, the company explained.

Biolinq’s CEO Rich Yang spoke to Medtech Insight about the company’s wearable patch in development, which uses tiny microsensors to measure, for now, glucose, with ample runway for additional indications down the line. If approved by the US FDA, the device would become the first of its kind to monitor glucose levels in diabetes patients not using insulin.

In this month’s Digital Health Roundup, Medtech Insight’s Marion Webb highlights AI discussions at the HLTH Europe conference and an interview with Motif Neurotech's CEO Jacob Robinson. Elizabeth Orr discusses DeepWell DTx's newly launched VR game for treating stress-related hypertension and anxiety. Natasha Barrow provides an overview of Digital Mental Health Technologies regulation in the UK and Brian Bossetta reports ‘the good and bad’ from Medtronic's report on digital technologies' use in the operating room.

Andrew Trister, Verily’s chief medical and scientific officer, spoke with Medtech Insight at the HLTH Europe conference about Verily’s newly launched Lightpath Metabolic solution, featuring GLP-1 prescription, AI and strengthened clinical support. Trister also talked about plans for the Study Watch and offered views on the Alzheimer’s research landscape and AI development and regulation in a new era of uncertainty.

This week, Boston Scientific agreed to pay $1B+ for stroke prevention device firm Silk Road Medical; the FDA asked for feedback on patient safety for non-device medical software; and a former medtech CEO was sentenced to six years for her part in a phony device scheme.

This week, AdvaMed and MITA win appeal to prevent repair companies from hacking medical devices, the FDA cleared Abbott’s Libre Rio CGM for OTC sales, J&J MedTech wins expanded clearance for Velys knee medical robot, the FDA updates its AI program, Canary Speech secures $13m in series A funding and Xeltis won FDA approval for an IDE submission to begin enrolling patients for a pivotal study for aXess.
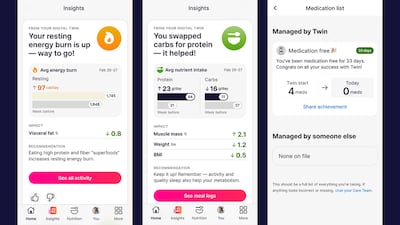
Twin Health extends its whole-body digital twin AI platform to help people who are overweight or obese achieve a healthy weight without the use of medications.

Dexcom launches its highly customizable One+ continuous glucose monitor in the UK as well as a state of type 2 report that suggests 63% of people living with type 2 diabetes have trouble managing their disease.
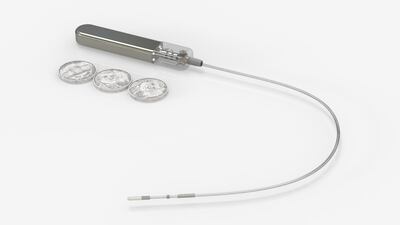
CGM company GlucoTrack Inc. recently announced plans to expand measuring glucose into the spinal epidural space for patients with painful diabetic neuropathy and its new computational data to support sensor longevity beyond three years from currently two years. Medtech Insight spoke with GlucoTrack’s CEO, Paul Goode, about the company's ambitions to offer more choice in glucose monitoring to people with diabetes.

The US FDA has cleared a glucose monitoring system from Senseonics. The authorization of the implantable insulin delivery system creates a new pathway for like devices.