
Switzerland

An agreement between the EU and Switzerland potentially opening the way for renewal of the MRA – enabling barrier-free cross-border medtech trade – could be concluded as early as December. More good news for Switzerland, relating to the acceptance of FDA-approved products into the country, could follow in Q1 2025.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 80 documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 30 documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 50 documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Sixty-five documents have been posted on the tracker since its last update.
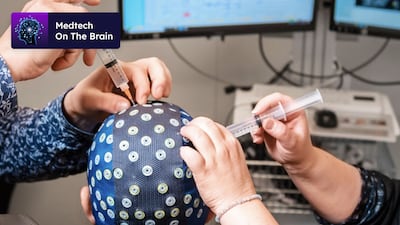
Swiss-based start-up DeepPsy aims to streamline mental health care using EEG and ECG biomarkers to better match depression patients with treatments. Co-founder Mateo de Bardeci discusses the company’s vision as it launches its in-house medical device as a service in Switzerland.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Thirty-five documents have been posted on the tracker since its last update.

Switzerland has been developing a national medical devices database to mirror Eudamed, the EU version that has excluded participation by Swiss companies since the failure of the federal council and European Commission to update the MRA for devices.

Negotiations aimed at renewing the close trading relationship and barrier-free mutual market access between Switzerland and the EU got underway on 19 March. Swiss medtech companies welcomed the move, stressing the need for the defunct mutual recognition agreement to be updated.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Thirty-six documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 50 documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.

Three central European industry associations have agreed to develop joint initiatives and form a collective voice with which to lobby decision-makers on medtech themes of mutual interest.

Medtech promotes more women to managerial roles than other industries, but senior leadership positions see a significant drop in women representation, indicating a "broken rung" higher up in the leadership ladder.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Forty-five documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.

Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Forty-three documents have been posted on the tracker since its last update.

A pilot trial of the swissdamed database – an initiative designed to mirror the EU’s Eudamed – was successfully completed in June, allowing the regulator Swissmedic to confirm live implementation in January 2024. Work on two swissdamed modules is underway.

The ongoing failure of Swiss and European Commission decision makers to resolve the issue of Switzerland’s institutional agreement is hampering EU market access for Swiss innovators like IVDs company Abionic. More pressingly, the EU must address the wider issue of the MDR’s negative impact on innovation and start-ups, says Abionic co-founder Iwan Märki.