
User Fees

Another record-breaking year for novel approvals looks out of reach, but the six novel agents with November goal dates show the continued strength of rare disease drug development.

The number of standard and priority reviews also decreased significantly in FY 2023 compared to the previous year, which caused the fee for redeeming a voucher to rise.

Advisory committee concerns cast clouds over Iterum’s oral antibiotic, Intercept’s Ocaliva, and perioperative immuno-oncology regimens, while CSL and Pfizer aim to take their hematology franchises in new directions.
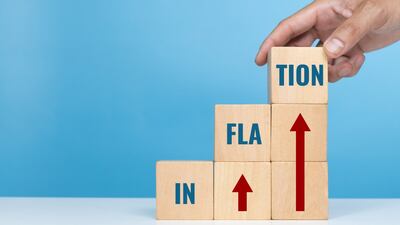
Inflation accounted for a larger portion of user fee revenue target increases for fiscal year 2025, compared to previous years, according to a Pink Sheet analysis.

FDA is considering allowing MIDD meeting requests to be submitted more often than the current quarterly schedule, but agency must have a plan to deal with the likely increased demand, official says.

The agency’s plan for advanced manufacturing seeks more harmonization, while also seeking to codify internal practices with guidance and training.

Teva requested the change to the draft guidance, arguing that otherwise the meetings would be “completely one-sided conversations.”

Pink Sheet infographic shows that while overall growth continues at the drugs and biologics centers, the US FDA still must add many employees to meet user fee-mandated hiring goals.

A lack of process documentation was part of the reason the FDA forgot to account for fee refunds in its estimates of incoming applications for two fiscal years, a mistake that likely cost the agency millions in revenue.

Pink Sheet reporter and editors discuss the US FDA drug approval decisions that could arrive in August, a trial design issue that could ensnare BMS’ Opdivo along with AstraZeneca’s Imfinzi, and the EMA potentially asking all sponsors to provide raw clinical data as part of application reviews.

A stacked user fee goal lineup sets the stage for market showdowns in primary biliary cholangitis and IgA nephropathy, the first psychedelic approval decision, and lots of targeted cancer therapies.

The agency used a 10-year average with updated figures to calculate the FY 2025 PDUFA application fee and limit the impact of submission volatility, but still allowed GDUFA and BsUFA fees to skyrocket.

But the agency should explain why different meeting types are granted than requested or denied entirely, industry representatives said during session on meeting management best practices.

US federal agencies are entering a new era of uncertainty and increased litigation over their regulatory interpretations, but the FDA may benefit from the unique circumstances that have evolved through user fee legislation.

US FDA review divisions will determine whether the meeting is appropriate because there are no one-size-fits-all requirements.
Old habits die hard when it comes to funding the FDA. When the agency is doing things well, it doesn’t get the money it needs to keep up the momentum.

With likely more fee-paying applications arriving at the agency, sponsors may be wondering whether user fees should have been increased as much as they were at the start of FY 2024.

FDA officials have said hiring could be slowed if an inflationary pay increase is not included in the agency budget, but CDER and CBER continue to add staff at a steady pace.

EMA charges participants to receive scientific advice through the fledgling program, unlike the US FDA, which may not fit the budgets of some complex generic sponsors. At the same time, sponsors also may simply not be aware the program exists yet.

US FDA’s drug center reported a net increase of more than 300 employees in FY 2023, while the biologics center saw an overall increase of more than 30.