
Patents
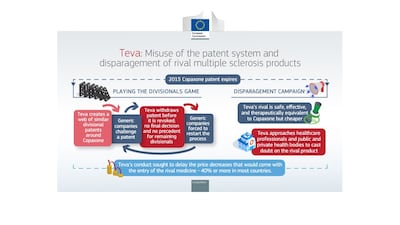
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.

A ruling by the Court of Justice of the EU has produced a clear definition of what constitutes the “first” marketing authorization when companies apply for SPCs on pharmaceutical products.

Divisional patents remain a major barrier to market entry for generics in Europe, heard attendees to Medicines for Europe’s legal affairs conference in Dublin earlier this month.

US Federal Trade Commission says the policy would enhance its ability to detect reverse payment settlements between pharmaceutical companies that raise antitrust concerns.
Industry groups say the initiative will undermine innovation and the competitiveness of European companies, impeding Europe's ability to tackle future crises effectively.

More legal action in India that has the Bolar exception provision at its crux with Roche, in this instance warding off a local drug maker in the Evrysdi patent infringement case.

The agency is also poised to announce another development in its scrutiny of the drug patents listed in the FDA’s Orange Book.

The rulemaking is supposed to formalize the Patent Trial and Appeal Board process, which has been in an interim status since a Supreme Court decision, giving manufacturers more ability to request oversight of the decisions.

The timing of the request, with Xtandi’s loss of exclusivity on the horizon, could be explained by the concern that a change in the US presidential administration in 2025 may limit the prospects for near-term relief.

Changes to inhaler pricing may be more ‘business as usual’ for the drug industry than the Vermont senator wants to let on. FTC, meanwhile, is keeping the patent pressure on Teva, the only inhaler manufacturer to not act following Sanders’ investigation.

Class action complaint alleges Boehringer Ingelheim engaged in a ‘Respimat Orange Book scheme’ to thwart generic competition for Combivent Respimat and Spiriva Respimat that cost payors millions, if not billions, in overcharges.

United Therapeutics Corp. claims the agency wrongly allowed Liquidia to submit an amendment to add a new indication to its tentatively approved NDA for a rival version of its pulmonary arterial hypertension blockbuster treprostinil.

A report from the European Commission highlights how the enforcement of pharmaceutical antitrust rules between 2018 and 2022 increased the availability of fairly priced innovative medicines in the bloc.