
United States

The former CDER Director spoke to the Pink Sheet about why a new statutory standard is needed for certain rare disease drug approvals. We lay out some of her thinking in part one of a multi-part series on the topic.

Many speakers at the Galien Forum were uncertain about changes the new Trump Administration could make the Inflation Reduction Act (IRA), National Institutes of Health funding and other areas.

The STAR pilot established in PDUFA VII for efficacy supplements, which was modeled after the popular Real Time Oncology Review program, has not garnered much interest. The agency now is establishing a separate but similar pilot for a small number of original NDAs and BLAs.

US FDA Commissioner Robert Califf offers some thoughts on improving the informed consent process during a Patient Engagement Advisory Committee meeting that reflect concerns informed consent documents are too long and complex, but also do not address everything that may be important to patients.
Plan sponsors continue working to offset the risk from major policy shifts.

Pink Sheet reporter and editors discuss potential changes that the incoming Trump Administration could make to the FDA, as well as worries about political interference in decision-making, and policy and staffing changes.

Former Trump adviser Tomas Philipson anticipates the FDA will undergo a deregulatory push during President Trump’s second term and emphasize speeding drugs to market.

Two more generics manufacturers have settled claims with 50 US attorneys general that they artificially inflated and manipulated the prices of generic drugs for nearly a decade.
Advertisers are facing a 20 November deadline to bring TV and radio ads into compliance, but stakeholders still question the reg’s scope, including whether and how it applies to ads on streaming services and social media platforms. FDA advisory comments suggest the agency is taking a hard stand on the rule’s ‘dual modality’ requirement.

Despite the recent anti-vaccine rhetoric in the final weeks of the Trump campaign, pulling an established safe and effective product off market would be difficult. But there’s little to stop political interference in approvals.

Drug pricing policies, M&A oversight, FDA and HHS leadership, the Biosecure Act and tariffs are among the issues pharmaceutical manufacturers will be closely watching under a second Trump Administration.

With Chair Lina Khan expected to exit the commission, the incoming administration may consider elevating one of the Republican commissioners on the panel to succeed her, at least on an acting basis.

While election results are not complete, prospects improve under new regime for 340B program reforms, PBM legislation, and Inflation Reduction Act drug pricing revisions. But immediate priorities for Congress and the new president will be elsewhere.

Increasing data flow into the agency could improve inspection decision-making.

An appeals court panel seemed skeptical of whether AstraZeneca has standing in its Administrative Procedure Act challenges against the IRA’s drug price negotiation program, but suggested a company with standing might be successful in their court.
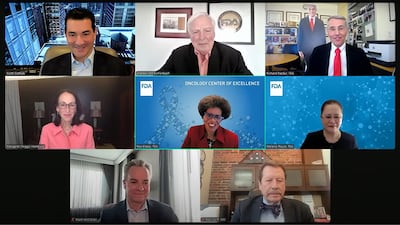
OCE Director Rick Pazdur celebrated 25 years at FDA by moderating a panel of five former commissioners. Topics included political challenges with the job and the ‘most memorable’ controversy they faced while in office.

PBM reform likely will remain a priority no matter which party controls Congress, but experts differ on the party that likely would be harder on the industry.

Two of three appeals court judges hearing Bristol Myers Squibb and Janssen’s appeal questioned whether Medicare’s drug price negotiation program was truly structured in a way that gives manufacturers a choice not to participate.

Former Food and Drug Law Institute CEO Amy Comstock Rick will take on patient engagement for the US FDA Rare Disease Hub as director of strategic coalitions.

US FDA’s Rare Disease Innovation Hub should shepherd the practical transformation of the diverse wealth of patient-generated data held by advocacy organizations into information for regulatory use, Reagan-Udall public meeting hears.