ADVERTISEMENT
Deals

Hikma says that its acquisition of Xellia’s finished dosage form business and a key contract manufacturing agreement in generics have kept its business on track to meet its full-year forecasts.

Meitheal has announced a licensing agreement with parent company Hong Kong King-Friend to add another three biosimilars to its pipeline for the US market.

Two more generics manufacturers have settled claims with 50 US attorneys general that they artificially inflated and manipulated the prices of generic drugs for nearly a decade.

The Denmark-based major intends to build on its heritage in diabetes and is keen to hear from innovators in a space where prevalence is on the rise but is not being served by improved new products.

A significant divestment in the works, complex generic launches, a mammoth antitrust fine in Europe – Teva’s Q3 was busy and bustling, led by strong, double-digit top-line growth and a further rise in guidance for 2024.

Zentiva has responded to recent rumors that owner Advent International may be looking to sell the firm in a deal worth as much as €5bn.

Two more generics manufacturers have reached agreements with 50 US attorneys general settling claims that they artificially inflated and manipulated the prices of generic drugs for nearly a decade.
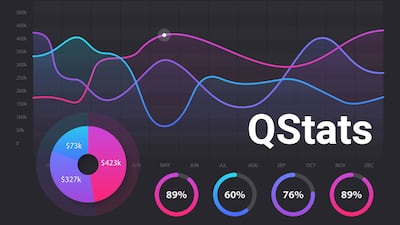
During Q3, biopharma merger and acquisition deal value reached $11.7bn and drew in $33.1bn in potential deal value from alliances. Device company M&A values reached $8.2bn, while in vitro diagnostics and research tools players’ M&A activity totaled $86.3m.

Unlike previous deals in this space which were mostly to do with discovering new compounds, the big pharma is paying $150m upfront to license a specific product.

During Q3, biopharmas brought in an aggregate $17bn in financing and device company fundraising totaled $3bn; while in vitro diagnostic firms and research tools players raised $1.1m.