
China

Learn more about how China’s biopharmas are going global by working with dependable and trustworthy companion diagnostics partners.

Low prices and intense competition for innovative drugs at home continue to drive Chinese biotechs' globalization. Broader domestic commercial health insurance might provide the key to change.

LaNova Medicines, as well as other Chinese RNA interference specialists and developers of stem cell-based therapies, were among those securing new funds from venture capital and private equity firms over the past few months.

The exact causes leading to Chinese authorities’ investigation of the UK major remain unclear at this point, but its key oncology drugs are facing fierce local competition from homegrown rivals.

High patient needs, a large healthcare market and innovation potential in the biotech sector in China still appear to be maintaining and attracting investments by overseas biopharma companies, including by Bayer and Lilly in biotech start-ups and Lilly in manufacturing.
ZL-1310, a DLL3-targeting ADC, has shown a 74% objective response rate in extensive-stage small cell lung cancer after platinum-based chemotherapy, making it a new contender in this setting.

Biostar plans to raise up to $41m through a Hong Kong IPO, which help the Chinese chemotherapy specialist progress lead asset utidelone for various oncology indications.

Dexter Yan talks about his September interview with Sharon Barr, AstraZeneca’s executive vice president, Biopharmaceuticals R&D. Hu Xu shares her articles on Sciwind’s ecnoglutide Phase III results and a Chinese law firm’s view on the proposed US BIOSECURE Act.

Sharon Barr, AstraZeneca’s executive vice president, biopharmaceuticals R&D, in an interview with Scrip, outlines how the company is using a holistic approach to define a new competitive landscape in the weight loss sector. She also sees new frontiers for the firm and its potential partners both in China and beyond to jointly explore aimed at maintaining healthy muscle mass while decreasing body fat.

Lilly’s Mounjaro to Pfizer’s Paxlovid, the WuXi group has been part of pharma majors’ sourcing chain. How big is the hole clients are to fill when the proposed BIOSECURE Act comes into effect? Here’s a Scrip infographic using Evaluate Pharma data.

Gilead’s divestment from a 50-50 joint venture with local partner, which came to light recently, will leave China’s cell therapies market under the total control of domestic players in the coming years.
Pipelines in bioconjugates continue to develop, from ADCs to XDCs, with radionuclide drug conjugates (RDCs) and antibody-oligonucleotide conjugates (AOCs) leading the expansion.

Without a placebo control arm, ZT002, a once-monthly GLP-1 receptor agonist from the Chinese biotech, reduced body weight by 17.1% from baseline at 30 weeks in Chinese obese patients, data from a Phase Ic clinical study showed.
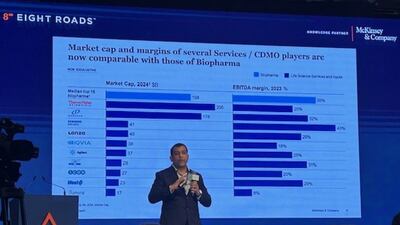
A redistribution of pharma outsourcing activities, both ex-China and also a wider shift in CDMO work from North America down the line may be in store. A senior McKinsey executive discusses with Scrip these trends in the CDMO sector amid geopolitical tensions, the spurt in customer queries at Indian firms and also facets of the deals scenario.
Interim results from the Phase III REMARK study presented at WCLC showed the Chinese company’s second-generation ALK/ROS1 inhibitor met its primary endpoint as a first-line therapy in ALK-positive advanced NSCLC.

Ivonescimab has shown significant and "extremely commercially meaningful" improvements in progression-free survival versus Keytruda as a first-line therapy for lung cancer in a trial in China, potentially positioning it as a new chemo-free solution for all PD-L1-positive patients in this setting.

CorrectSequence led the pack of Chinese cell and gene therapy developers seeking new funding with a roughly $14m series A-plus round. In other modalities, antibody-focused developers Mabgeek and Novamab closed series Bs.

Akeso's CEO has highlighted favorable hazard ratio results for the Chinese biotech's anti-PD-1/VEGF bispecific antibody ivonescimab in a head-to-head trial with Keytruda across Chinese NSCLC patients with positive PD-L1 expression in a first-line setting.

China's Duality Biologics, founded in 2020, has developed two-thirds of its clinical-stage antibody-drug conjugates by combining antibodies acquired from domestic peers with its proprietary linker-payload platform.

The nod will help unlock a national market for the partners’ Dupert (fulzerasib) in which roughly 50,000 people are expected to be newly diagnosed with lung cancer harboring the KRAS G12C mutation in 2025. Meanwhile, two other homegrown rivals are also closing in on marketing clearances in China.