
India

Ex-Janssen India chief and BSV CEO Sanjiv Navangul shares with Scrip glimpses of his life and professional journey including chasing the IAS dream early on, the heady days at Hoechst with a stint in Moscow, challenges around Sirturo’s debut and unprecedented preps that fueled Januvia’s big run during his innings at MSD.

Novo Nordisk India MD talks to Scrip about what to expect when Wegovy arrives in India and the local trajectory of Rybelsus. The Danish group also shared findings of a study around obesity.

Lanreotide supply challenges could impact Cipla’s US revenues in the coming quarter, while the firm hopes it can seize emerging opportunities in the GLP-I segment in India, where Novo Nordisk and Eli Lilly are progressing filings for their blockbuster products.

Strides is to supply semaglutide and liraglutide to generics companies amid global capacity constraints and drug shortages, ahead of loss of exclusivity. An ability to make drug-device combinations has meant it has contracts for seven of the eight GLP-1 agonists, and counting

CEO of Sumitomo Pharma America unit, Adele M. Gulfo, draws from experiences working across companies including Pfizer, to advise on go-to-market strategies for a portfolio across primary care to rare disease.

In a video interview, Nandini Piramal, chairperson of Piramal Pharma, speaks with Scrip about a focus on on-patent products, an integrated ADC offering, a measured approach to the proposed BIOSECURE Act and the company’s strategy to double revenue to about $2bn

A court order encompassing funding, drug pricing, clinical trials and overall policy implementation aspects is expected to bring about a paradigm shift in the treatment of rare diseases in India. Sarepta, Roche and Sanofi are among the key players that have been part of pricing discussions.

Indian CMOs, CDMOs and CRDMOs are expanding capacity and making leadership moves ahead of the BIOSECURE Act, anticipating demand from big pharma and budding biotechs. Scrip brings details on leading Indian firms which could be potential go-to suppliers for US companies affected by the proposed Act

Syngene’s CEO talks to Scrip about how the firm is deepening capabilities in areas like ADCs, PROTACs and why the US Inflation Reduction Act may not necessarily shrink outsourced small molecule work. The executive also shares his view on the China ‘rebalancing’ trend amid geopolitical tensions and the US BIOSECURE Act.

Gilead strikes voluntary licensing agreements with six generic manufacturers for lenacapavir but health groups seek clarity on pricing and seeming API supply restrictions. All eyes are also on the patent opposition case in India.

A high-profile panel that included a senior executive from F-Prime Capital, Novo Nordisk's India chief and a leading endocrinologist discussed, at a recent event, some of the big opportunities, challenges and transformational trends underway as obesity drugs take the world by storm.

GSK’s India chief talks to Scrip about new category creation in adult immunization with Shingrix seeing strong uptake, Nucala and Trelegy’s trajectory and oncology assets set for debut as the company steps into its 100th year of operation in the country. GSK expects to continue to transform its operating model as things evolve, he indicates in the concluding part of this interview.

GlaxoSmithKline Pharmaceuticals’ MD tells Scrip how the company has been able to reinvent itself, contemporize brands and get supply chain innovation right as the UK-headquartered firm marks 100 years of operations in India. GSK’s equivalent of the 'Amazon model' for vaccine ordering and the new temples of industry are some of the other facets the executive discussed in the first instalment of this two-part interview.
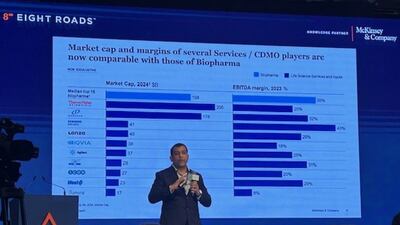
A redistribution of pharma outsourcing activities, both ex-China and also a wider shift in CDMO work from North America down the line may be in store. A senior McKinsey executive discusses with Scrip these trends in the CDMO sector amid geopolitical tensions, the spurt in customer queries at Indian firms and also facets of the deals scenario.

Human milk could have a role in preventing Alzheimer’s disease and other ailments linked to the gut microbiome, says former Pfizer VP and Hilleman CEO Davinder Gill. He also talks about a halal meningitis vaccine, and tailwinds for an oral cholera vaccine now launched by Bharat Biotech in this fascinating interview

Kamil Hamied, son of Cipla’s outgoing vice-chair M K Hamied, is set to re-join the company as non-executive director, following his "entrepreneurial journey." Does it signal a shift in the promoter group’s immediate considerations amid past stake sale speculation or mark routine top-level executive changes?

"Illegal" copies of Pfizer’s Lorbriqua and Eli Lilly’s Verzenio have been seen making their way to India allegedly via manufacturers in Bangladesh. The US multinationals are engaged in a legal battle in India to tackle the ‘infringement’ amid wider concerns around inadequate regulatory oversight.

A licensing deal for Leqvio (inclisiran) is all in a day’s work for Mankind which also recently acquired Bharat Serums and Vaccines at a valuation investors were initially not entirely pleased with. As the youngest company in the top five of the domestic pharma market sets a 5X revenue target, what could help it get there and what’s to watch out for?

Does Zydus’ 50% stake acquisition in Sterling Biotech reflect a broader move of formulations companies to secure API supplies or acceleration of API segment restructuring begun by a couple of private equity (PE) funds?

Indegene’s CTO talks to Scrip about the state of GenAI implementations in pharma amid hype and limited clarity on ROI, the need to address foundational issues early on, promising pilots, the firm’s alliance with Microsoft and Google’s generalist LLM for therapeutics.