ADVERTISEMENT
Review Pathway
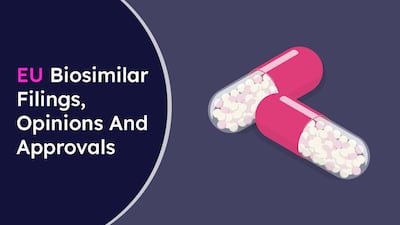
A list of EU biosimilar filings, CHMP opinions and EU marketing authorizations, including details of the biosimilar company, the brand name/INN, indication(s), the reference product/company, and the date and type of event.
Swissmedic has removed the requirement for companies to participate in accelerated application hearings in certain situations.

At the advisory committee review of Stealth’s Barth syndrome treatment elamipretide, Office of New Drugs Director Peter Stein clarified the circumstances where clinical data in a related indication could serve as confirmatory evidence for a single adequate and well-controlled study.

US FDA Office of New Drugs Director Peter Stein says review divisions have made the case that a discussion-only meeting would solicit the necessary input.

Manufacturers looking to change their device sterilization method as part of the move away from ethylene oxide (EtO) may be able to make use of the Predetermined Change Control Plan (PCCP) process, FDA officials said in a webinar this week.

Nipocalimab is an investigational FcRn blocker for treating generalized myasthenia gravis that was also recently filed for regulatory review in the US.

The number of standard and priority reviews also decreased significantly in FY 2023 compared to the previous year, which caused the fee for redeeming a voucher to rise.

A US FDA webinar for start-up sponsors showcased staff who sound enthusiastic about helping drug developers advance their projects, while also emphasizing the importance of early and continual engagement with the agency.

US FDA Office of New Drugs Director Peter Stein says review divisions have made the case that a discussion-only meeting would solicit the necessary input.
Aeon Biopharma has heralded its latest interaction with the US FDA over its planned Botox biosimilar as a win that sets the stage for analytical studies, trials and a subsequent filing.