ADVERTISEMENT
US States

Beachbody offers supplement sales using health savings accounts; PlantX, Boka launches oral health supplements; supplements take Form from RTE meal provider Factor; LIV3 JV behind marketing SugarShield; Ghost among Keurig Dr Pepper brands; and Nutra-Med Packaging expands with Legacy Pharma.

New Jersey Assembly passed bill amended to expand extensively in defining which products, or ingredients, would be subject to a ban on sales to consumers younger than 18.

California authorities say predominance of retailers licensed to sell hemp are compliant with ban published effective on 3 October through emergency regulation. But hemp industry’s contesting the regulation in state court, arguing state authorities inappropriately used emergency action to change state law on hemp products legislature passed in 2021.

The Pink Sheet highlights recent comments and insights from pharma officials and executives on key issues we are covering.

The Washington State Department of Ecology will try to work with companies that violate the Toxic Free Cosmetics Act, rather than reflexively imposing the $5,000-per-violation fine for first-time offenders, says the law’s implementation planner. She noted financial assistance is available for small businesses, as well as incentives for companies adopting measures “beyond compliance.”
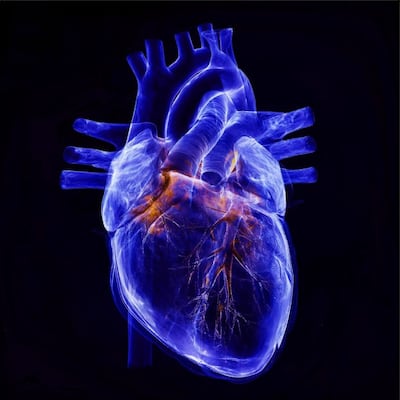
Tricuspid valve innovation has taken off since the US FDA’s 2023 authorizations of Edwards' Evoque and Abbott's TriClip systems. Whether to repair or replace tricuspid valves remains an open, nuanced question among cardiologists. Dr. Henrik Treede of University Hospital Mainz and TriCares CEO Ahmed Elmouelhi offer views on the evolving space.

Toxic-Free Cosmetics Act planner Shari Franjevic acknowledges the Washington State Department of Ecology is authorized to use enforcement discretion regarding the new law’s 1ppm limit on trace lead in cosmetic products, effective 1 January 2025, or raise the ceiling via rulemaking. But it does not seem inclined to do either based on data known to the department at present.

CRN/Radicle Trailblazing Woman Award to ChromaDex’s Yasmeen Nkrumah-Elie; Nourish’s TV ad campaign features US soccer star Alex Morgan; Vitamin Shoppe BodyTech Elite Altered Strength line sponsors Team Red, White & Blues; contract pharma/consumer health product development firm LabConnect appoints Wesley Wheeler CEO; Avanos’ Game Ready is “Recovery Partner” of NFL’s Nick Bosa; Akita adds Robert Hanson to advisory board; and BODi has wrestler as brand ambassador

Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.

After launching Retaine MGD Advanced, OcuSoft says a release by Bruder Healthcare referenced Retaine MGD trademark and statements from a previous OcuSoft announcement about the original product attributed to an optometrist. B+L, Rohto brand and homeopathic firm Relief Products also make US OTC eye care space moves.