ADVERTISEMENT
Medical Device

Behind the scenes, there were many conflicting, but also useful discussions, which ultimately shaped the message the European Parliament delivered to the European Commission in late October about the need for changes to the Medical Device and IVD Regulations.

An interactive look at recent executive-level company changes and promotions in the biopharma, medical device and diagnostics industries.
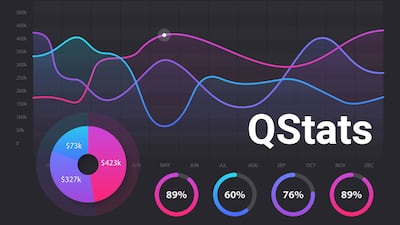
During Q3, biopharma merger and acquisition deal value reached $11.7bn and drew in $33.1bn in potential deal value from alliances. Device company M&A values reached $8.2bn, while in vitro diagnostics and research tools players’ M&A activity totaled $86.3m.

During Q3, biopharmas brought in an aggregate $17bn in financing and device company fundraising totaled $3bn; while in vitro diagnostic firms and research tools players raised $1.1m.

An interactive look at medtech and diagnostics deals made during August 2024. Data courtesy of Biomedtracker.

The Design for Life roadmap will help medtech companies comply with the UK NHS’s Net Zero 2045 greenhouse gas emissions target. A dedicated medtech innovation center is mooted.

CE marking for its Accu-Chek Smartguide continuous glucose monitor means Roche is on the cusp of challenging existing major players in Europe with a device whose predictive algorithms can help sufferers plan and prepare longer into the future.

A new UK medtech survey sets out industry’s market access and regulatory concerns and makes clear where system users see the need for improvement. There are some grounds for optimism, but the MHRA’s planned rise in regulatory fees could undo some of the good work.

The US FDA’s Office of Women’s Health provides a research roadmap to address health concerns specific to women. The FDA recently updated the roadmap, outlining areas in which further research is needed.
