Interviews
IMU Biosciences joins a UK consortium of 27 academic and industry partners to undertake “immunoprofiling” of 6,000 cancer patients to assess their response to treatment.

In this week’s Digital Health Roundup, Medtech Insight’s Marion Webb talks about her interview with GE HealthCare’s chief AI officer Parminder Bhatia about his vision for AI in health care and other highlights from HLTH. Natasha Barrow discusses her interview with Owkin on the EU AI Act and highlights Click Therapeutics’ latest clinical results.
The company has taken the ambitious approach of establishing a manufacturing base capable of producing millions of devices before any attempt at a regulatory submission.

Patients treated with Abbott’s Esprit BTK system had better results and fewer repeat procedures after two years than a control group, the company announced this week. Abbott vascular leader Jennifer Jones-McMeans spoke with Medtech Insight about what the results could mean for patient care.

Medtech Insight talked with GE HealthCare’s chief AI officer Parminder “Parry” Bhatia at HLTH about the firm’s new CareIntellect for Oncology offering to help clinicians make efficient use of multimodal patient data, his vision for projects within AI Innovation Lab, and the future of AI in health care.

University of Cambridge's soon-to-be spun-out Nimble Genomics is among grant-winning projects in Capital Enterprise's Cancer Tech Accelerator. Medtech Insight spoke to clinical oncologist and Nimble founder Henno Martin and co-founder Radek Lach to learn more what about the brain cancer blood test’s prospects in an increasingly competitive liquid biopsy space.

After more than 20 years in patient care, cardiologist Ethan Korngold became chief medical officer for Abbott’s vascular business in April. How was his first conference on the industry side of the table? “Incredibly invigorating,” he said, offering updates on Abbott’s Ultreon and Esprit BTK product lines.
Craig Eagle, chief medical officer of Guardant Health, provides his view on an increasingly competitive liquid biopsy market in this interview with Medtech Insight.

The co-founder of Pure Global discusses the regulatory consultancy's use of AI to support clients’ marketing submissions and other needs, as well as the AI-enabled medtech landscape and opportunities in China and Southeast Asia for accessing patient data for AI development purposes.

Medtech Insight spoke with Vicky Demas, CEO of Identifeye, about plans for bringing the company’s AI-powered retinal screening system for early detection of diabetic retinopathy to primary care facilities. More than 50% of the roughly 38 million Americans who have diabetes skip retinal screenings at present, increasing risk of developing the leading cause of blindness in adults.

The College of American Pathologists wants a US district court to scrap the FDA’s final rule that unilaterally assumes oversight of lab-developed tests, but not because the college feels the agency has no business regulating them. Helena Duncan, senior director of quality at CAP, explained the college’s position to Medtech Insight.
Tricuspid valve innovation has taken off since the US FDA’s 2023 authorizations of Edwards' Evoque and Abbott's TriClip systems. Whether to repair or replace tricuspid valves remains an open, nuanced question among cardiologists. Dr. Henrik Treede of University Hospital Mainz and TriCares CEO Ahmed Elmouelhi offer views on the evolving space.

Richard Lowenthal, co-founder and CEO of ARS Pharmaceuticals, highlights the crucial unmet need for needle-free devices to treat type 1 allergic reactions, given challenges associated with current epinephrine injectors. Hear what’s next for the Neffy intranasal spray and ARS Pharmaceuticals.

Predetermined change control plans have potential to accelerate US FDA review timelines for device modifications, but visibility remains low as to whether the program is behaving as advertised and delivering efficiencies sought by sponsors. Joshua Oyster, partner in Ropes & Gray’s Washington, D.C. office, offers perspective.

Patrick Alexandre, Crossject CEO, discusses crucial developments happening for Zeneo, a needle-free injector, functioning intramuscularly to administer medication in a tenth of a second.
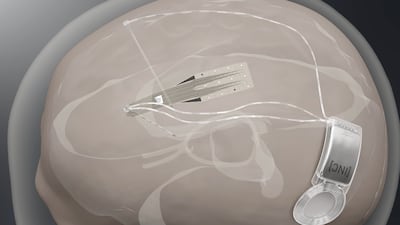
Spain-based Inbrain Neuroelectronics plans first-in-human study to show safety of its graphene-based technology in direct contact with human brain while also developing a second interface for treating Parkinson’s disease.
Genetic Analysis CEO Ronny Hermansen and Christina Casén, senior VP of clinical and medical affairs, discuss the company’s polymerase chain reaction (PCR)-based approach to gut microbiota profiling versus DNA sequencing, competitive landscape, and opportunities for supporting pharma R&D and assessing drug treatment success.

The ISRCTN clinical trials registry has launched an improved dashboard to provide metrics that reveal how many studies are complying with key transparency requirements. Badges are in place for individual studies meeting the transparency criteria.

Altoida CEO Mark Jones has high hopes that the company’s digital assessment tool will be approved by the FDA to be used along with blood biomarker testing by primary care doctors to help predict Alzheimer’s disease before patients show symptoms.

Altoida CEO Marc Jones spoke with Medtech Insight about the company’s investigational digital screening tool for Alzheimer’s and the dire need for better, more accessible precision neurology diagnostics as the global population ages, neurologist shortages worsen, and groundbreaking Alzheimer’s drugs change the treatment paradigm.