Manufacturing

CRO executive Horst Ruppach discusses the critical factors that drug manufacturers must consider to ensure compliance with the revised ICH Q5A(R2) guideline on biologics viral safety. Thorough documentation and proactive engagement with regulatory bodies are key to navigating the complexities of viral safety evaluation in biotechnology products, he says.

Increasing data flow into the agency could improve inspection decision-making.

Cell and gene therapy manufacturers based in Europe should speak to local regulators to understand how to demonstrate compliance with EU-level good manufacturing practice guidelines, as each country will apply the rules differently, an expert explains.
The UK government has introduced draft legislation that will regulate the manufacturing of innovative medicines such as cell and gene therapies at the point of care. The country’s medicines regulator, the MHRA, says the framework is the “first of its kind in the world.”

Whether the legislation aimed at discouraging use of Chinese contractors passes in its current form or not, industry will continue to face more pressure to decouple its operations.

Pink Sheet reporter and editors discuss an emerging pharma strategy to avoid Medicare price negotiations, legal wrangling related to compounding GLP-1 drugs for obesity and diabetes, and the varying opinions of FDA officials on the acceptability of artificial intelligence models that are not fully explainable.

FDA law experts do not buy the outsourcers’ argument that the agency must go through notice-and-comment rulemaking to remove a drug from the shortage list.

Decentralized manufacturing methods for cell and gene therapies will be critical for improving patient access to treatments, but sponsors must prepare to demonstrate “comparability” with centralized manufacturing.

New guidance from the European Medicines Agency explains how in vitro and in vivo models may be used instead of clinical data for the purpose of establishing therapeutic equivalence in a stepwise approach.
The US FDA Commissioner pushed for rebalancing the US’s pharmaceutical supply chains while also stressing that US-China commerce has a role that would be risky to compromise.

With BIOSECURE's legislative progress on pause until after the election, a Pink Sheet infographic looks back on the Capitol Hill progress to date and looks ahead to the potential impact if it is enacted, using Evaluate Pharma data to highlight the likely holes in pharma’s supply chain.

Improvements in the UK’s manufacturing and clinical trial ecosystems are on the “Christmas list” of Ioana Parsons, Ipsen’s general manager for the UK & Ireland.

Regulators in Europe and the US have demonstrated a commitment to providing guidance that will make decentralized and point-of-care manufacturing a reality, the CSO of GermFree, a company that provides mobile and modular cleanrooms for manufacturing advanced therapies, tells the Pink Sheet.
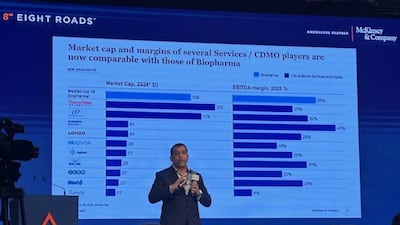
A senior McKinsey executive discusses trends in the CDMO sector amid geopolitical tensions, the spurt in customer queries at Indian firms and also facets of the deals scenario.

With the BIOSECURE Act halfway to congressional approval, stakeholders are pushing for a financial boost to ensure the US gains the business Chinese companies will lose, but that may be a big lift.

The revised EU Urban Wastewater Treatment Directive, which obliges pharmaceutical and cosmetic industries to contribute at least 80% towards the costs of removing micropollutants from wastewater through quaternary treatments, will place an additional burden of around €2bn per year on German manufacturers, says Pharma Deutschland.

The agency’s plan for advanced manufacturing seeks more harmonization, while also seeking to codify internal practices with guidance and training.

CBER Director Peter Marks said recent leaps in gene therapy science have not been matched by more affordable manufacturing technology and that the field needs to set a cost-effectiveness target.

The World Health Organization says its new 98-page guidance dealing with wastewater and solid waste management for antibiotic manufacturing is a first of its kind and addresses an important but neglected issue.

The deadline is nearing for pharmacopoeias to express their interest in joining the decades-old discussion group that works to harmonize excipient monographs and general chapters and reduce the burden on manufacturers to perform analytical procedures in different ways depending on the jurisdiction.