Policy & Regulation

Consumers in Denmark no longer need a prescription to buy opioid antagonist naloxone as the country looks to tackle the growing issue of overdose.

To improve engagement with patient information, a UK ePI Task Force comprising PAGB, ABPI and BGMA is looking at how electronic patient information leaflets (ePILs) can be introduced in the country.

Proposed administrative order likely is first to prompt opposition from OTC industry or other stakeholders potentially delaying or deferring the agency’s expectation for moving its proposal to a deemed final order effective one year after it would be published.

“The infant formula business is recovering and we've taken actions to simplify and consumerize our business, but there's a lot more work to do,” says CEO Patrick Lockwood-Taylor as Perrigo’s announced latest results.
Whether Democrat Harris or Republican Trump is in White House or which party has majority in either chamber of Congress, the FDA’s conundrum will continue around a regulatory pathway for allowing use of ingredients derived from hemp in supplements.

The International Council for Advertising Self-Regulation has launched a "Global Think Tank" to further effective industry self-regulation in the area of advertising, backed by national authorities such as the UK's ASA.

Although FDA concluded OTC oral phenylephrine is ineffective as a nasal decongestant in doses up to highest studied level, federal preemption precludes challenges to agency’s regulation of labeling for phenylephrine and other Rx and OTC drugs, according to recent decision in New York federal court.

Perrigo is among the supporters of a campaign to get a clause of Germany's Medicines Advertising Act removed, which bans the advertising of the OTC morning after pill.
Supreme Court’s Loper Bright “decision is central to this case,” says Jonathan Emord, attorney representing Alliance for Natural Health USA and Meditrend Inc. in a complaint filed in US District Court for the District of Columbia.
FDA’s OTC office director details a 2023 guidance as opening doors for NRT innovation at recent public meeting, but researchers and an industry executive note the most recent approval in the US for an innovative NRT was more than 20 years ago and say FDA isn’t allowing sufficient flexibility for approvals of new products or indications other than cessation related to quitting smoking.

FDA tobacco programs chief emphasizes moving smokers to lower risk alternatives and NIDA executive encourages proposals for e-cigarettes as nicotine replacement treatments. FDA also seizes $76m in unauthorized e-cigarettes.

Nevertheless, the problematic text of the political declaration, which industry argues incorrectly implies that the United Nations General Assembly is advocating for a restriction on sales of commonly used non-prescription antimicrobials, such as topical antifungal thrush and foot creams and antiviral cold sore treatments, was approved.

Biden’s order for proposed rules could boost support for Democrat Kamla Harris from undecided voters concerned about reproductive rights or for Republican Donald Trump from those who agree with his conservative stance on access to birth control, which he argues should be up to states to regulate. Proposed rules also could stir consumers not planning to vote to go to the polls.
“Politicians must create the right framework” to promote Rx-to-OTC switch in Germany, insists Pharma Deutschland deputy general manager Elmar Kroth.

Qnovia notes NRT inhalation product recently received investigational new drug clearance from FDA as agency and NIH say innovation needed smoking cessation to help improve rate of success for quitting the habit that kills around 500,000 US consumers annually.
Slow adoption of alternatives to animal testing in the current decentralized regulatory framework.

Requests for “enforcement actions are not within the scope of FDA’s citizen petition procedures,” CDER says, rejecting petition dosing device firm Parenteral Technologies submitted as it prepares for workshop on Pediatric Research Equity Act requirements for OTC NDA sponsors.
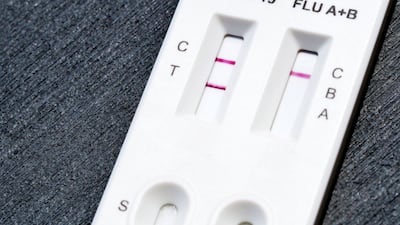
FDA grants de novo authorization to Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first OTC flu test to be cleared outside the emergency use pathway.

UK pharma also reaches agreement in principle, subject to DoJ approval, to pay $70m to resolve a whistleblower complaint filed by Valisure, the testing lab which in 2019 raised concerns about a potential link between the use of drugs containing ranitidine, a histamine-2 blocker, and cancer.

NDAs for additional OTC products containing acetaminophen and/or NSAIDs and indicated for use by children between 2 and less than 12 years old would trigger compliance by application sponsors with the act passed in 2003 to address lack of pediatric use information in drug labeling.