Regulation

Consumers in Denmark no longer need a prescription to buy opioid antagonist naloxone as the country looks to tackle the growing issue of overdose.

Proposed administrative order likely is first to prompt opposition from OTC industry or other stakeholders potentially delaying or deferring the agency’s expectation for moving its proposal to a deemed final order effective one year after it would be published.

Although FDA concluded OTC oral phenylephrine is ineffective as a nasal decongestant in doses up to highest studied level, federal preemption precludes challenges to agency’s regulation of labeling for phenylephrine and other Rx and OTC drugs, according to recent decision in New York federal court.

Perrigo is among the supporters of a campaign to get a clause of Germany's Medicines Advertising Act removed, which bans the advertising of the OTC morning after pill.
Supreme Court’s Loper Bright “decision is central to this case,” says Jonathan Emord, attorney representing Alliance for Natural Health USA and Meditrend Inc. in a complaint filed in US District Court for the District of Columbia.
FDA’s OTC office director details a 2023 guidance as opening doors for NRT innovation at recent public meeting, but researchers and an industry executive note the most recent approval in the US for an innovative NRT was more than 20 years ago and say FDA isn’t allowing sufficient flexibility for approvals of new products or indications other than cessation related to quitting smoking.

FDA tobacco programs chief emphasizes moving smokers to lower risk alternatives and NIDA executive encourages proposals for e-cigarettes as nicotine replacement treatments. FDA also seizes $76m in unauthorized e-cigarettes.
Slow adoption of alternatives to animal testing in the current decentralized regulatory framework.

Requests for “enforcement actions are not within the scope of FDA’s citizen petition procedures,” CDER says, rejecting petition dosing device firm Parenteral Technologies submitted as it prepares for workshop on Pediatric Research Equity Act requirements for OTC NDA sponsors.
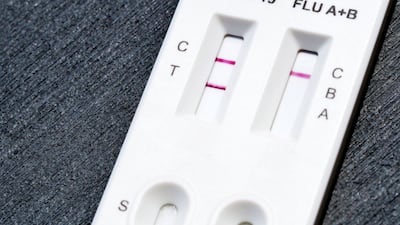
FDA grants de novo authorization to Healgen Rapid Check COVID-19/Flu A&B Antigen Test, making it the first OTC flu test to be cleared outside the emergency use pathway.

UK pharma also reaches agreement in principle, subject to DoJ approval, to pay $70m to resolve a whistleblower complaint filed by Valisure, the testing lab which in 2019 raised concerns about a potential link between the use of drugs containing ranitidine, a histamine-2 blocker, and cancer.

NDAs for additional OTC products containing acetaminophen and/or NSAIDs and indicated for use by children between 2 and less than 12 years old would trigger compliance by application sponsors with the act passed in 2003 to address lack of pediatric use information in drug labeling.

Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.

Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”

Nonbinding forecast, fourth for the program, includes risks associated with codeine-containing cough medicine as a topic FDA will include in ongoing evaluation of GRASE for pediatric cough cold drug products marketed under monograph for cold, cough, allergy, bronchodilator and anti-asthmatic OTC drugs.
Council for Responsible Nutrition contends New Jersey bill, which Assembly Health Committee amended with substantial language to make restrictions more stringent on 23 September, is targeted as inaccurately as restrictions in New York effective in April.
October will see the bricks-and-mortar launch of the first FDA-approved OTC treatment for erectile dysfunction in the US by Haleon. HBW Insight catches up with the company's North America president Lisa Paley to find out what Haleon has planned for Eroxon's arrival in stores.

Following Energy and Commerce Health Subcommittee hearing about FDA’s human food and tobacco programs on 10 September, gap between what the trade groups, committee leadership and the FDA each want more of doesn’t appear to be shrinking.

This Thursday (26 October) the United Nations will discuss whether OTC antimicrobial products should be restricted to prescription-only status by UN member states. In advance of this, we catch up with UK association CEO Michelle Riddalls, to learn about the PAGB’s role in crafting the global consumer healthcare industry’s response.

FDA approves Flumist consumers can use at home for prevention of flu caused by virus subtypes A and B in individuals 2 through 49 years old in a proposal submitted by AstraZeneca’s MedImmune.